Abstract
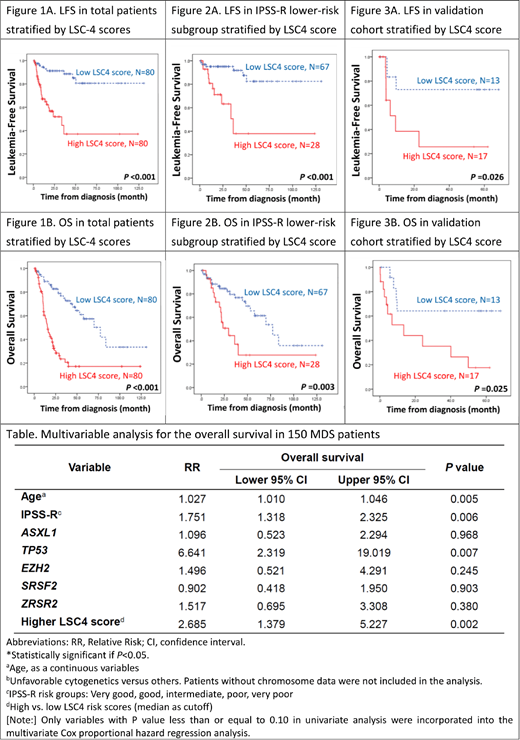
Leukemic stem cells (LSCs) possess biological properties shared with normal hematopoietic stem cells. They are responsible for chemoresistance and relapse in acute myeloid leukemia (AML). Although myelodysplastic syndrome (MDS) has traditionally been regarded as a "hematopoietic stem cell disorder", the clinical and biological impact of LSCs on MDS patients are not well defined. To address this question, we used the Affymetrix HTA 2.0 microarray platform to profile 16 out of the 17 recently reported stemness genes (one of them, the ARHGAP22 gene was not included in our array) in our 160 adult primary MDS patients. The diagnoses were based on the 2016 World Health Organization (WHO) classification. Patients with antecedent chemotherapy or hematologic malignancies were excluded. Forty-one (25.6%) patients had MDS with single lineage dysplasia (MDS-SLD), 20 (12.5%) had MDS with ring sideroblasts (MDS-RS), 31 (19.4%) had MDS with multilineage dysplasia (MDS-MLD), 32 (20%) had MDS with excess blasts-1 (MDS-EB1) and 36 (22.5%), MDS-EB2. The risk distribution of the cohort was very-high risk, 15.3%; high risk, 21.3%; intermediate risk, 24.7%; low risk 34.7%; and very-low risk 4% according to the revised international prognosis scoring system (IPSS-R). We identified 4 genes (LAPTM4B, NGFRAP1, NYNRIN, and EMP1) whose expression levels were significantly correlated with overall survival (OS). An LSC4 score (0.731 x LAPTM4B - 1.259 x NGFRAP1 + 0.304 x NYNRIN + 0.231 x EMP1) was constructed based on the weighted sums derived from Cox regression analysis. Higher LSC4 scores were associated with higher IPSS-R scores, complex cytogenetics, and higher incidences of mutations of RUNX1, ASXL1, TP53, SRSF2 and ZRSR2. High-score patients had significantly higher 3-year AML transformation rate (36.3% vs 11.3%, P<0.001), shorter leukemia-free survival (LFS, median, 33.6 months vs not reached, P< 0.001, Figure 1A), and shorter OS (median, 15.9 months vs 77.3 months, P<0.001, Figure 1B). In subgroup analysis, LSC4 score could also well stratify MDS patients with normal karyotype into different risk groups (p<0.001). The prognostic significance of LSC4 scores for LFS, AML transformation rate, and OS still remained valid in both IPSS-R lower-risk (very low, low, and intermediate risk) subgroup (Figures 2A and 2B) and higher-risk subgroup (high and very high risk). In multivariate analysis, higher LSC4 score was an independent adverse risk factor for OS, irrespective of age, IPSS-R, and mutation status of ASXL1, TP53, EZH2, SRSF2, and ZRSR2 (Table). We also well validated the prognostic significance of the LSC4 scores in an independent cohort of 30 MDS patients (Figures 3A and 3B). Interestingly, patients with higher LSC4 scores had a better OS if they received allogeneic transplantation than those who did not. It implied that transplantation might ameliorate the poor survival impact of high LSC4 scores. In conclusion, through comprehensive analysis, we have created a simple and powerful LSC4 scoring system, which serves as an independent prognostic factor for OS and LFS in MDS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal